Policy & Regulation: Page 2
-
The silver lining around Lilly’s Alzheimer’s delay could be a future with better drugs
Although a regulatory delay for Eli Lilly’s donanemab in Alzheimer’s disease is a competitive setback, researchers have their eye on better drug development as a result.
By Michael Gibney • March 12, 2024 -
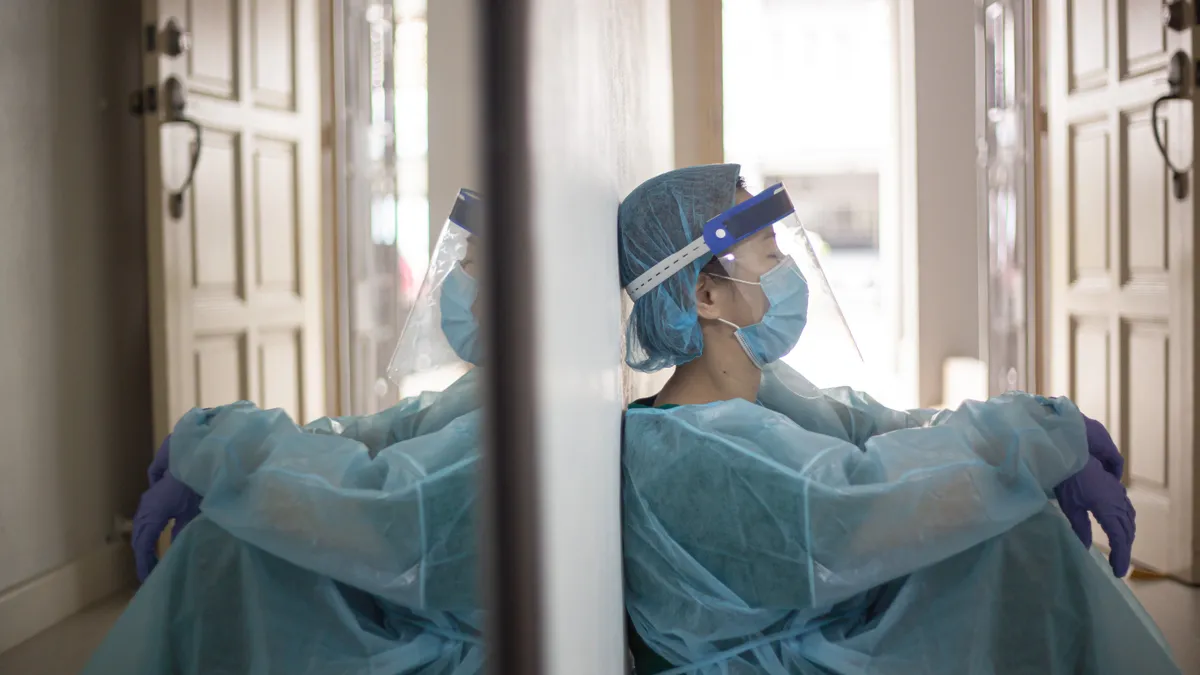
Amylyx ALS drug fails crucial study, putting company’s future in doubt
The results have led Amylyx to pause promotion of Relyvrio and potentially pull it from the market in the coming weeks, a major blow to the company and ALS patients.
By Jacob Bell • Updated March 8, 2024 -
 Explore the Trendline➔
Explore the Trendline➔
 Stock via Getty Images
Stock via Getty Images Trendline
TrendlineEmerging biotech
After years of financial turmoil, the biotech market is turning the page. Strong fundamentals, including solid clinical data, are key for biotechs hoping to capitalize on the improving conditions.
By PharmaVoice staff -
Could pharma’s legal attack on the IRA succeed in killing price negotiations?
An all-out blitz across U.S. courts is bolstering pharma’s goal to overturn Medicare drug price negotiations.
By Amy Baxter • March 6, 2024 -
Profile
HIV isn’t ‘solved,’ but a doctor who treated some of the first patients hopes to finally deliver a cure
From San Francisco in the 80s to a gene therapy prospect, Dr. Marcus Conant looks back on his long fight against the virus — and if the industry is close to ending the epidemic.
By Michael Gibney • March 5, 2024 -
Did the FTC get it wrong when it blocked the Sanofi-Maze deal?
In its zeal to stamp out anticompetitive practices and lower drug prices, a consultant said the FTC might have overlooked the unique considerations in rare disease drug development.
By Alexandra Pecci • March 4, 2024 -
After ‘tragic’ bacterial outbreak, lawmakers press FDA to step up foreign inspections
Impatience to fix long-held challenges to overseas drug facility inspections is growing on Capitol Hill.
By Amy Baxter • March 1, 2024 -
How a 15-year-old Genzyme drug shortage became a legal smorgasbord of pharma’s thorniest issues
Rare disease treatments, drug shortages, a market monopoly and march-in rights all play a role in the story of newly revived litigation against Sanofi’s Genzyme.
By Alexandra Pecci • Feb. 26, 2024 -
Behind the breakthrough cancer therapy that just won a historic FDA nod
The first-of-its-kind TIL therapy for solid tumors developed by Iovance Biotherapeutics won FDA approval last week.
By Kelly Bilodeau • Feb. 21, 2024 -
Biden or Trump: How deeply will 2024’s election outcome impact pharma?
The election is still months away, but campaign promises are solidifying, and signal the benefits and drawbacks of each candidate for pharma.
By Michael Gibney • Feb. 20, 2024 -
FDA inches closer to defining its regulatory role in AI
Commissioner Dr. Robert Califf signals how the FDA will consider AI in drug development and how the agency may use the emerging technology in the future.
By Amy Baxter • Feb. 12, 2024 -
Astellas’ science chief on the leap into new technologies
After a big 2023, Yoshi Shitaka said the company is banking on gene therapies, KRAS degraders and more to keep the momentum.
By Meagan Parrish • Feb. 9, 2024 -
‘Beginning of the end’ for small molecules? VCs brace for Medicare investment gap
The "small molecule penalty" becomes even riskier for investors as the IRA negotiates prices, and VCs and lawmakers are looking to get a jump on those headwinds.
By Michael Gibney • Feb. 6, 2024 -
Government hits patent thickets on multiple fronts
Patent thickets are the latest target in lawmakers' bid to lower drug prices.
By Alexandra Pecci • Feb. 5, 2024 -
Pfizer’s COVID vax and antiviral sales have crashed. Will the ‘big bet’ on Seagen even the keel?
In the first earnings call since the $43 billion Seagen deal closed, CEO Albert Bourla stressed that Pfizer is all in on the promise of ADCs to make up for COVID losses.
By Michael Gibney • Feb. 1, 2024 -
CAR-T developers respond to FDA black box warning
Despite a recent safety warning from the FDA, companies such as BMS and J&J stress that the benefits of their cancer therapies still outweigh the risks.
By Kelly Bilodeau • Jan. 30, 2024 -
AstraZeneca, Daiichi aim for first pan-tumor ADC approval
A “tumor agnostic” FDA approval for the companies’ Enhertu would break new ground for antibody-drug conjugates.
By Ben Fidler • Jan. 29, 2024 -
The biggest questions hanging over pharma in 2024
Key trends on the horizon in the life sciences this year.
By Meagan Parrish • Jan. 25, 2024 -
Breast cancer tops list of most studied diseases
The most studied diseases last year included three types of cancer as research into COVID-19 fell sharply in the post-pandemic era.
By Kelly Bilodeau • Jan. 24, 2024 -
FDA looks to execute ‘largest reorganization in history’ this year
What started as an examination of the Human Foods Program has expanded into an agencywide overhaul.
By Alexandra Pecci • Jan. 23, 2024 -
How to make 2024 a banner year for biopharma, despite the headwinds
Creative thinking is the name of the game as biopharmas seek to overcome a host of challenges — and investing with purpose, building trust and embracing tech could get them there.
By Michael Gibney • Jan. 18, 2024 -
An enduring COVID mystery: Why some do fine and others die
The infectious disease market could be shifting toward a future not only focused on the microbe, but on the unique genetic attributes of the host.
By Alexandra Pecci • Jan. 17, 2024 -
FDA chief says agency can’t manage health tech alone
FDA Commissioner Dr. Robert Califf recently spoke about the future of AI and machine learning and the need for constant vigilance to improve healthcare systems.
By Alexandra Pecci • Jan. 16, 2024 -
4 big FDA approval dates to watch in 2024
Following a year where the FDA approved 55 new drugs, the 2024 PDUFA calendar is set with a number of potentially buzzy approvals.
By Alexandra Pecci • Jan. 12, 2024 -
Opinion
Pharma execs sound off on trends and policies they’re tracking in 2024
From regulatory concerns to leadership skills, pharma leaders at JPM shared how they’re approaching the coming year.
By Meagan Parrish • Jan. 12, 2024 -
Opinion // Year in Preview
PharmaVoice’s Crystal Ball: What’s next in drug innovation and clinical trials
As advanced therapies and patient perspectives strengthen their foothold in R&D, pharma is poised to deliver impactful treatments in record time.
By Meagan Parrish • Jan. 11, 2024