The Enhertu effect: Daiichi’s plan to build on the success of its crown jewel
Right now is “singularly, the most exciting moment” in Daiichi Sankyo’s 100-year history, said Ken Keller, CEO and president of the company’s U.S. business and head of its global oncology unit.
Riding high from the success of the AstraZeneca-partnered blockbuster cancer medication Enhertu, the company in October announced a partnership with Merck & Co. valued up to $22 billion. And with potential new approvals coming this year, Daiichi is on track to become a cancer powerhouse ahead of its own schedule.
Born from a 2005 merger between two long-standing Japanese pharmas — Sankyo Co. and Daiichi Pharmaceutical Co. — the company has been “cardio-centric” for most of its history, Keller said. Now the second largest pharma in Japan, its diversified portfolio also includes vaccines, migraine meds and more.
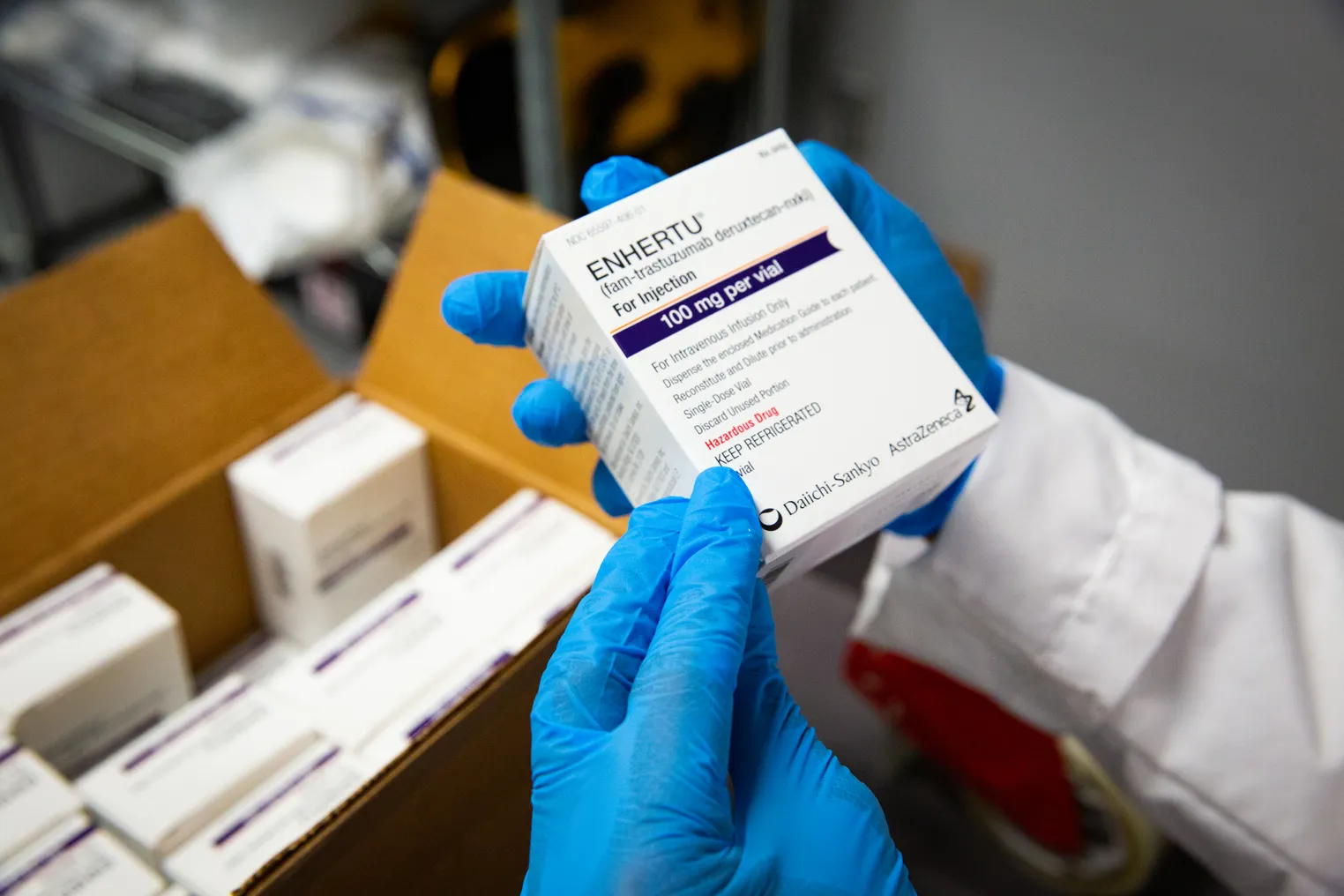
But Daiichi trained its eyes on oncology in 2020 when it launched Enhertu for HER2-positive unresectable or metastatic breast cancer, vowing to become a “top 10 cancer pharma company” within a decade.
“That was a big ambition coming from zero,” Keller said. “Here we are, five years later, and we’ve changed how cancer is treated.”
The company’s special sauce is a proprietary antibody-drug conjugate platform called DXd it used to develop six candidates for multiple cancers. Since an original nod in breast cancer, Enhertu has racked up approvals for several other HER2-related indications, including the first tumor-agnostic approval for an ADC, which the FDA granted in April.
Daiichi and AstraZeneca, its development and commercialization partner for Enhertu, also recently announced positive topline results from a phase 3 trial in patients with lower levels of the HER2 protein. The results, which the companies said showed a “statistically significant and clinically meaningful benefit” in progression-free survival, could help move the ADC into earlier lines of care.

Meanwhile, the company has three key PDUFA dates within nine months. This June, the FDA will render a decision on a HER3 treatment for non-small cell lung cancer that is now a part of its three-drug collaboration with Merck. The agency is also slated to announce decisions for a TROP2-directed ADC Daiichi is developing with AstraZeneca — first in NSCLC this December and then for breast cancer in January 2025.
If the approvals are granted, Keller said Daiichi will be poised to hit “top 10 cancer company status” by fiscal year 2026.
It’s got plenty of competition. With ADCs becoming one of pharma’s hottest commodities, Pfizer scooped up ADC specialist Seagen in a $43 billion acquisition last year. But Daiichi is slated to dominate the ADC market through 2029 with the highest level of sales, according to analysis by GlobalData.
Here, Keller discusses growth prospects for Enhertu and how ADCs could one day become curative treatments in cancer care.
This interview has been edited for brevity and style.
PHARMAVOICE: Sales for Enhertu more than doubled last year from about $1.25 billion in 2022 to $2.5 billion. Tell me about the long-term prospects for the drug.
KEN KELLER: Today we’re looking at five indications that we’ve received. We announced a positive pivotal trial, which would be another indication [and] a pretty amazing potential indication in the HER2 low space. The study looked at using it to displace chemotherapy rather than after chemotherapy, and we also demonstrated efficacy in the ultra-low HER2 setting.
When you look at breast cancer, HER2 positive is about 20% of cases, HER2 low is about 50%, and including ultra-low means we can help close to 90% of women with breast cancer. It would be the biggest drug ever in breast cancer. That gives you a sense of where the program is going.
Enhertu has been massive for Daiichi, but is the company relying too heavily on this single drug for future growth?
Enhertu is the foundation of everything we’re doing. Today we’ve got the ADCs we’re developing with Merck and AstraZeneca, we have two other DXd treatments in the clinic and we are leveraging our leadership in ADCs. So, we’ve got second and third generations of treatments we’re working on right now.
We’ve got a decade of clear growth, and hopefully our technology continues to grow and improve.
Do you see any new, big partnerships on the horizon?
It’s going to come down to the specific molecule or ADC we’re talking about. If we have a drug that we think has just a few indications, we can do that ourselves. If we have a drug that we think can be used across a dozen different cancers, that’s when we think about how we can develop it as fast as we can, and get it to as many patients as we can.
I’ve been here about 10 years and was the first employee of the oncology unit. Back in 2015, we had one oncologist — today we have over 2,000 people in the oncology part of our business. We are much more capable today, but we know we have to be open to working with others.
One of the biggest criticisms of emerging technologies in oncology is that pharma companies are making blockbusters out of drugs that only add months to patients’ lives. Do you have development efforts underway aimed at improving outcomes for ADCs?
This is what makes this DXd platform so unique. In our first indication [for Enhertu] we compared it to Kadcyle from Genentech, and we didn’t add months to survival — we added years. In second-line breast cancer, we added 28 months of progression-free survival compared to six.
When I look at starting an oncology franchise within a company, you couldn’t find a more profoundly beneficial drug. But two things are going to make a difference going forward.
We are going to move into early-stage disease and catch it earlier with a potent drug.
The second is moving into a combination setting with immuno-oncology treatments like Keytruda. We started in late-stage as a monotherapy, and as we move earlier and look at the power of ADCs and immuno-oncology, we think that could change things.
In the earlier stage and in the adjuvant or near adjuvant setting, a cure is the goal.